Virtually all processes in living cells are dependent on protein–protein interactions. Recent advances have highlighted the significance of amyloidogenic interactions occurring between proteins, resulting in the formation of amyloids, that are characterized by the presence of stacked b-strands that are organized perpendicular to the fibril axis. Amyloidogenic protein-protein interactions occur during the oligomerization process leading to pathological fibril formation, potentially allowing a functional role in the regulation of biological processes by specific oligomeric amyloid folds.
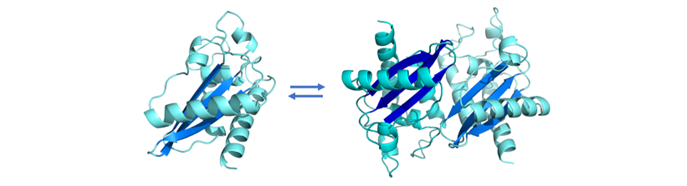
We previously showed that Golgi-Associated plant Pathogenesis Related protein 1 (GAPR-1) acts as a negative regulator of autophagy by interacting with Beclin 1 at Golgi membranes in mammalian cells. Both GAPR-1 and Beclin 1 have amyloidogenic properties and using a novel screening assay we uncovered and characterized the amyloidogenic interaction between GAPR-1 and Beclin 1. This research provides novel tools for interference with autophagy.
GAPR-1 is part of the CAP superfamily of protein that al share a common CAP domain. The amyloidogenic properties of the CAP domain suggest that the CAP domain may regulate amyloidogenic protein-protein interactions of all CAP family members. Proteins of the CAP superfamily have been implicated in a plethora broad diversity of biological functions, including immune defense in plants and mammals, prostate and brain cancer, sperm maturation and fertilization, fungal virulence, the regulation of extracellular matrix and branching morphogenesis, and toxicity of insect and reptile venoms.