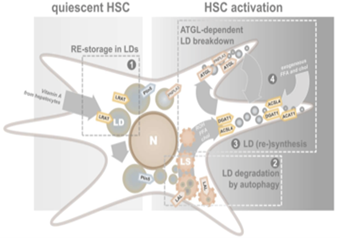
Hepatic Stellate cells (HSCs) have gained recognition for their dynamic, multifaceted roles in liver health and disease. HSCs, also referred to as Ito cells, fat-storing cell, star-like cells, or lipocytes, account for 5–15% of the total liver cells. In a healthy liver, quiescent HSCs are specialized in the storage of retinol (ROH; vitamin A) as retinyl esters (REs). After liver injury, HSCs lose their characteristic LDs and transdifferentiate into myofibroblasts (activated HSCs) that secrete retinoids, inflammatory components, and components of the extracellular matrix (ECM).
https://www.mdpi.com/2073-4409/9/10/2244
HSC activation is a critical step in the development of chronic liver disease and HSCs are the primary source of liver cancer-associated fibroblasts (CAFs) in HCC and liver metastasis. Thus, a detailed molecular understanding of the transition of quiescent HSCs to activated HSCs can offer novel insights into pathogenic mechanisms and guide targeted therapeutic strategies for various liver conditions.
We investigate the activation process of HSCs using primary cells in combination with different culturing conditions, allowing to study the quiescent and activated phenotype, as well as the transition phase. Using lipidomic analysis and flux studies with heavy-isotope labeled fatty acids, we have identified different metabolic lipid droplet pools in HSCs and elucidated the molecular machinery (DGAT1, ATGL, ACSL4) for the incorporation of polyunsaturated fatty acids in a dynamic lipid droplet pool that appears during their activation. A different molecular machinery operates in the preexisting “original” lipid droplet pool of quiescent HSCs and we recently showed that the production of retinyl esters by lecithin:retinol acyltransferase (LRAT) is sufficient for the biogenesis of these lipid droplets.